

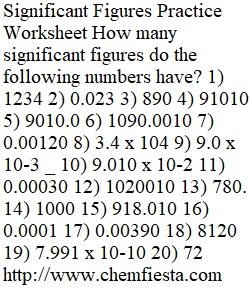
Q Significant Figures Practice Worksheet How many significant figures do the following numbers have? 1) 1234 2) 0.023 3) 890 4) 91010 5) 9010.0 6) 1090.0010 7) 0.00120 8) 3.4 x 104 9) 9.0 x 10-3 _ 10) 9.010 x 10-2 11) 0.00030 12) 1020010 13) 780. 14) 1000 15) 918.010 16) 0.0001 17) 0.00390 18) 8120 19) 7.991 x 10-10 20) 72 http://www.chemfiesta.com Kuta Software - Infinite Algebra 1 Writing in Scientific Notation Name Date Period Write each number in scientific notation. 1) 0.000006 2) 5400000 3) 60 4) 0.009 5) 6.7 6) 0.0000002 7) 2000000 8) ?? ? ??? 9) 48900 10) 0.0000009 11) ???? ? ??? 12) ?? ? ???? 13) 0.000216 14) 0.0042 15) ???? ? ???? 16) 4.8 Write each number in standard notation. 17) ??? ? ???? 18) ? ? ???? 19) ? ? ??? 20) ??? ? ??? 21) ???? ? ??? 22) ??? ? ???? 23) ???? ? ???? 24) ??? ? ??? 25) ??? ? ??? 26) ???? ? ??? 27) ??? ? ???? 28) ?? ? ??? 29) ??? ? ???? 30) ? ? ??? 31) ??? ? ??? 32) ? ? ???? Name:______________________ Period #:___ Temperature Conversion Worksheet Formulas: F arenheit to Celsius Co = 5/9(Fo-32) C elsius to Farenheit: Fo = 9/5 Co +32 C elsius to Kelvin Ko= Co + 273.15 K elvin to Celsius: Co= Ko - 273.15 Use the formulas above to convert the temerpatures to different scales. Convert the following to Fahrenheit Convert the following to Celsius 1) 10o C 6) 32o F 2) 30o C 7) 45o F 3) 40o C 8) 70o F 4) 37o C 9) 80o F 5) 0o C 10) 90o F 11) 212o F Convert the following to Kelvin 12) 0o C 13) -50o C 14) 90o C 15) -20o C Convert the following to Celsius 16) 100o K 17) 200o K 18) 273o K 19) 350o K ? Practice Unit Conversions Complete the following problems (in the space provided) by showing all of your work - and by drawing a box around your final answer. “showing all of your work” means setting up the entire equation and using unit abbreviations for each value 1. 3 meters into centimeters 2. 10 kilometers into meters 3. 15,050 milligrams into grams 4. 3,264 milliliters into liters 5. 9,674,444 grams into kilograms 6. 3.1 kilograms into milligrams 7. 5,897,159 milligrams into kilograms Questions 1-7 were conversion problems within the metric system. Questions 8-13 are unique conversion problems because they are asking you to convert between two different systems: the English System and the Metric System As long as the English system continues to be used, conversions between the two systems will be necessary. Use the conversion factors below to complete the problems that follow. Length Volume Mass 2.54 centimeter = 1 inch 1 liter = 1.06 quarts 1 kilogram = 2.20 pounds 1 meter = 3.28 feet 3.79 liters = 1 gallon 1 meter = 1.094 yards 1.609 kilometer = 1 mile 8. 4.5 inches into centimeters 9. 25.3 meters into feet 10. 2.3 miles into kilometers 11. 14 inches into centimeters 12. 125 pounds into kilograms 13. 20 gallons into liters Name Density Practice Problem Worksheet 1) A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density? 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. 3) What is the weight of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL. 4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? 5) A flask that weighs 345.8 g is filled with 225 mL of carbon tetrachloride. The weight of the flask and carbon tetrachloride is found to be 703.55 g. From this information, calculate the density of carbon tetrachloride. 6) Calculate the density of sulfuric acid if 35.4 mL of the acid weighs 65.14 g. 7) Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL. 8) A block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm. The block weighs 1587 g. From this information, calculate the density of lead. 9) 28.5 g of iron shot is added to a graduated cylinder containing 45.50 mL of water. The water level rises to the 49.10 mL mark, from this information, calculate the density of iron. 10) What volume of silver metal will weigh exactly 2500.0 g. The density of silver is 10.5 g/cm3.
View Related Questions